The organic chemistry class is a staple of the junior year medical society courses, like the cat dissection and structure function lab.
“The goal of the class is to give students some introduction to the topic before they go off to college,” explained to Mrs. Sanchez.
The class acts as a precursor gives insight into one of the most rigorous college courses in the United States. Although the class took a little while to get started, starting in the spring instead of the fall, Mrs. Sanchez taught the first organic Chem class of 2018 on Tuesday the April 10.
This class is intro to what organic Chem entails, including organic nomenclature, (naming the structure of molecular structures). This was mostly a review for the class because naming molecular structures was one of the cornerstone subjects for sophomore year Chemistry. I personally found it nice to go over a subject I had mostly forgotten. For those that might be confused, naming molecular structures looks something like.
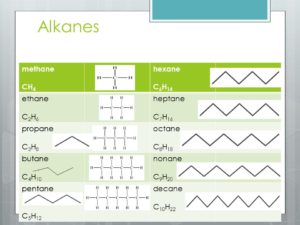
Organic Nomenclature
The prefixes for naming are meth, eth, pro, but, pent, hex, hept, oct, non, and dec. The -ane suffix refers to the carbons that make up the parent chain. Another fun rule about the carbons is their octet/valence shell, that must be filled up with 4 hydrogens or other atoms. Therefore, on methane, one carbon must have 4 hydrogen or other atoms to have a full octet. However, when carbons branch off of the parent chain, like so,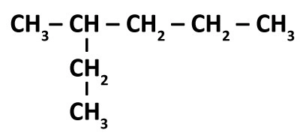
it leads to a new addition to the name, using the prefix meth, eth, etc, and the suffix -yl, making new names such as 3-methylhexane.
The 2 references the carbon number on the parent chain that the chain is attached to. For really long carbon chains with lots of branches, we can get lots of fun names like 2,3,4-triethyl-1-propylcyclopentane. In this case, the term cyclopentane refers to a five carbon long chain in a pentagon shape with the mother chain starting on 1, with a 3 carbon branch. Then the name refers to the two carbon long branches on carbon numbers 2,3, and 4, on the mother chain. Fun Right?
Mmm Cinnamon…
Over the past two meetings, we have done a lab where we isolate the cinnamaldehyde from cinnamon sticks. We did this by crushing the sticks into little slices and mixing them with water inside of a reaction vessel. We then placed the vessel in boiling water for 25 minutes. Once the time was up, we extracted the cinnamaldehyde by mixing the solution with ethyl ether.
Next, we dried the cinnamaldehyde with magnesium sulfate and placed it under a hood for about 5 minutes (the room smelled really cinnamony at this point). After the time ran out, we mixed our dried cinnamaldehyde with ammonium hydroxide and sodium hydroxide, to produce a clear liquid with our isolated cinnamaldehyde coating the outside of the vessel. All of this might sound like a lot of work to do for no extra credit or bonus of any kind but the people in the class have their own reasons for taking the course.
Vaughn Camp: “I wanted an intro course to the subject so when I got to college it wouldn’t be so difficult.”
Marco Campioli: “Because its a class and I want to take all the classes.”
Eduardo de Leon: “It is a good intro to the laboratory side of of chemistry.”
This is just a little taste of what the sophomores have to look forward to next year. Make sure to stay tuned to the Roundup for the latest Medical Society news.






